FDA
What will TNKase’s 5-second IV bolus mean for EMS stroke care?
There are two general causes of oxygen regulator fires, adiabatic heating and particle ignition
Albuterol, fentanyl and naloxone injectable are among the drugs being recalled – many of which are in very short supply, with no alternate source available
The Lucira COVID-19 & Flu Home Test can differentiate and detect influenza A and B
An EF3 tornado damaged the Pfizer factory responsible for producing nearly 25% of the company’s sterile injectable medicines
Senate Bill 186 allows the use of Opvee as overdoses in the state are rising
Studies, funded by the federal government, found Opvee achieved similar recovery results to Narcan
FDA Commissioner Dr. Robert Califf cited “the dire public health need” for the opioid antagonist
The overdose treatment could be more widely available in spring
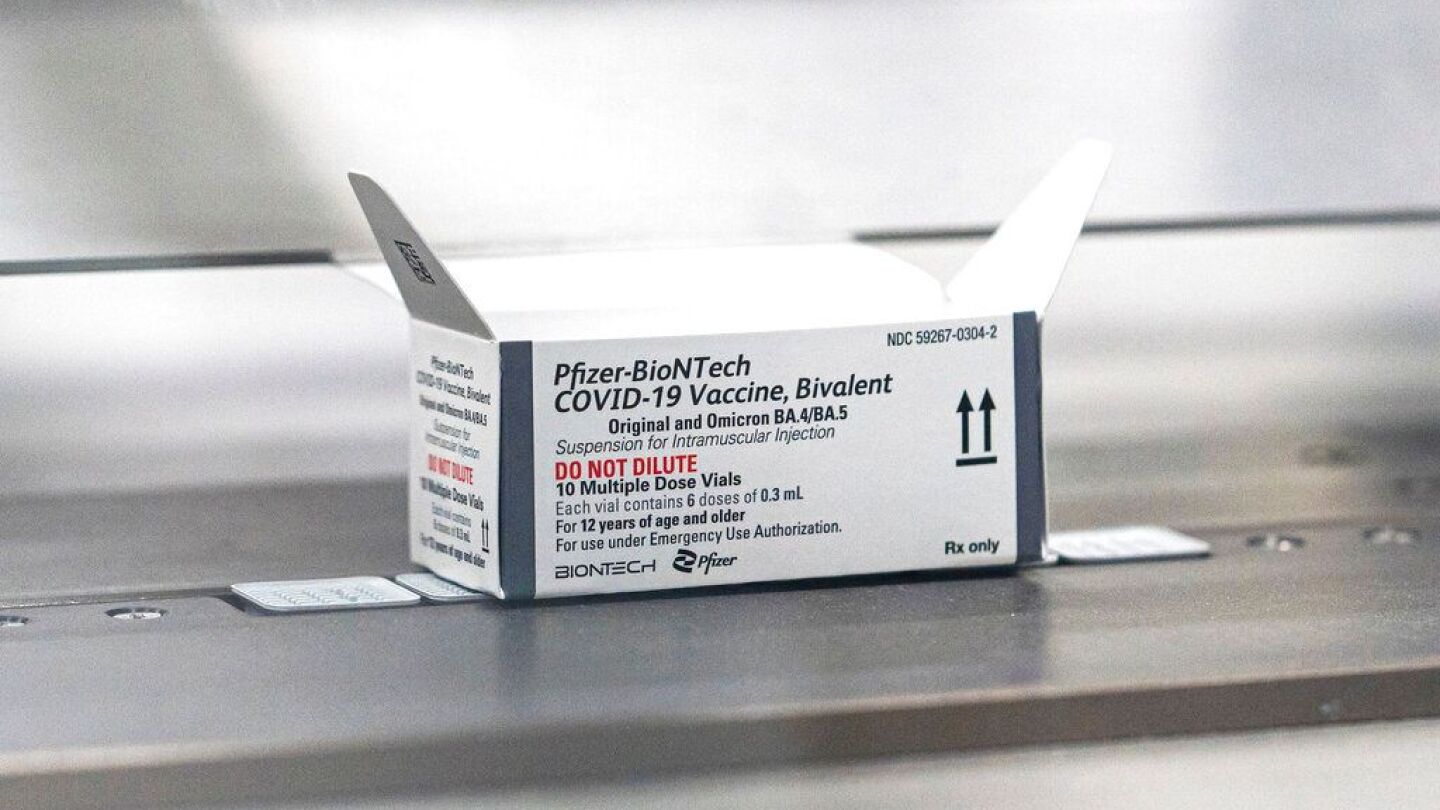
The hope is that the bivalent shots will thwart another winter surge
Robert Califf layed out a framework for combating drug addiction and overdoses
The CDC will issue its recommendation on vaccine administration after an upcoming vote
The CDC will decide whether to formally recommend the booster for this age group
Paxlovid is a faster, cheaper treatment than other authorized drugs, but initial supplies will be extremely limited
The treatment has been shown to significantly lower the rates of hospitalizations and deaths among people with coronavirus infections
The push comes amid concern about increased spread of the virus from holiday travel and gatherings
The CDC will consult with experts before finalizing official recommendations for who should get boosters and when
An independent expert panel will publicly debate the evidence on October 26 for Pfizer and BioNTech’s Emergency Use Authorization application
Patients who received the molnupiravir pill within five days of symptom onset had about half the rate of hospitalization and death as patients on the placebo pill
The emergency use authorization for the monoclonal antibody therapy has been expanded to include prevention for those exposed to COVID-19
A study found that kids between 12 and 15 developed higher levels of antibodies than young adults in earlier studies
The federal agency recommended using one mask per patient due to an increased PPE supply
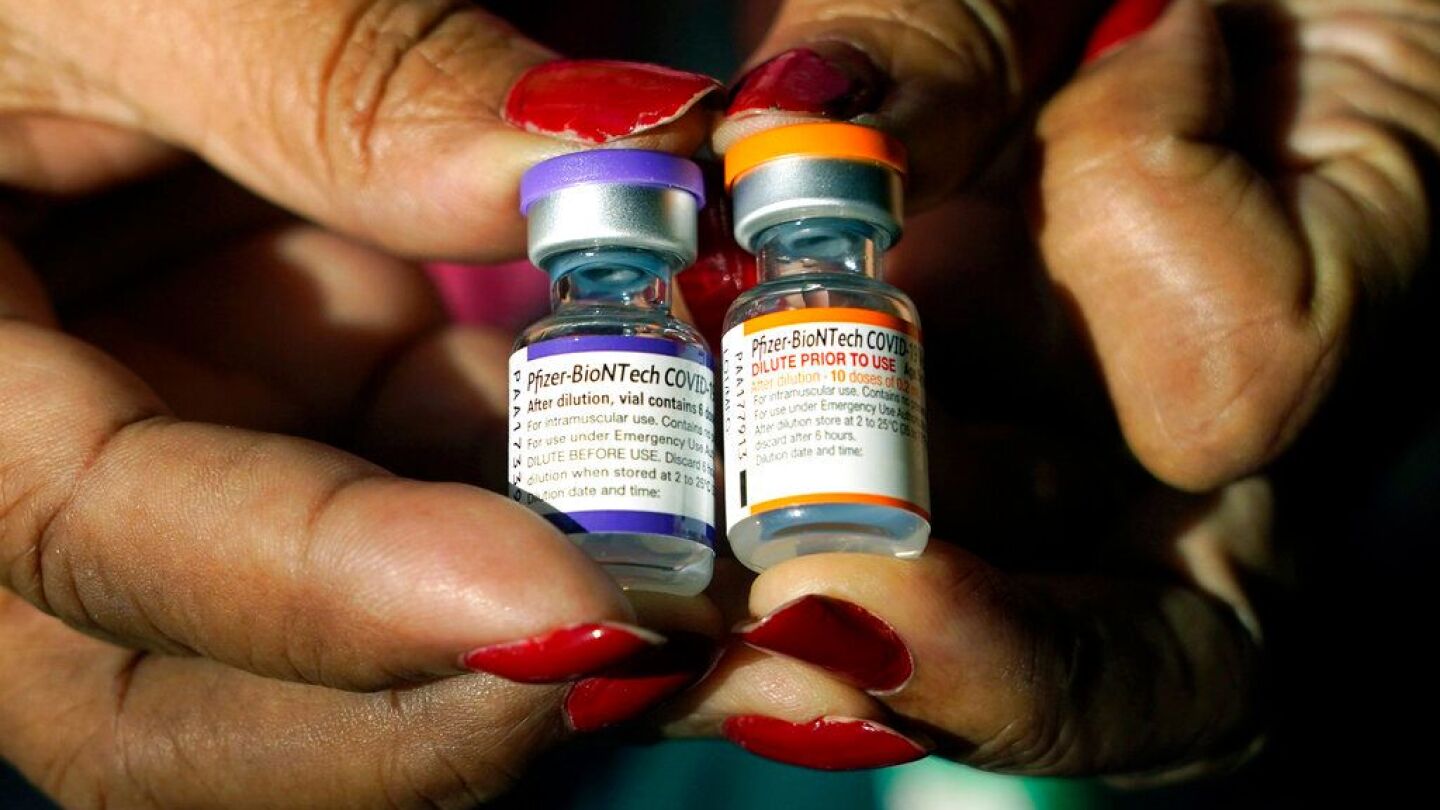
The agencies said they were investigating unusual clots in six women that occurred 6 to 13 days after vaccination
The glucagon comes in a 1 mg vial as part of an emergency kit
The one-shot vaccine may help speed distribution efforts if it is approved by federal regulators
An advisory panel is expected to vote to recommend the second vaccine on Thursday
Deliveries of the Pfizer vaccine are scheduled throughout Monday and Tuesday to healthcare facilities and nursing homes across the country
The move sets off what will be the largest vaccination campaign in U.S. history